
ST420,
Breakthrough Treatment
For Endometriosis
SUMMARY
ST420, a breakthrough disease-modifying therapy for endometriosis and other chronic immuno-inflammatory diseases
Strong preclinical efficacy and safety POC, 18 months to IND
Large unmet medical need, underserved market
Seasoned team of scientific, regulatory and business leaders
Best-in-class, novel, proprietary small molecule
Multi-Bn$ global sales potential
Clear investment opportunity at the steepest inflexion point

ABOUT US
The Apikal Therapeutics team: seasoned scientific and business leaders
A world-class Scientific Ad Board is currently being assembled. CMO / CDO and Program Management Officer to join as soon as initial funding is obtained
Stéphane Thiroloix CEO
GSK, BMS, IPSEN EVP Corp Dvt, Smith & Nephew Europe, Mayoly Spindler CEO, AlgoTx Founder & CEO.
Stéphane joined Apikal in July 2025. He is a pharma veteran with 20 years in C-suite roles. In his previous role as Founder & CEO of AlgoTx, Stéphane raised 35 M€ over 5 years, assembled a lean, world-class executive and non-executive team and took its core asset from concept to Phase 2.
Executive team


Guillaume Canaud (MD, PhD, HDR)
Co-founder, Sc. Advisor
Prof of Nephrology.
Head of Translational & Targeted Therapy Unit, University Paris Cité
Research Director INSERM
PI3K / AKT / mTOR expert

Laurent Micouin (PhD, HDR)
Co-founder, Sc. Advisor
PhD Organic Chemistry
Research Director CNRS
Director, Laboratory of Pharmacological and Toxicological Chemistry & Biochemistry, University Paris Cité,

David Schilansky
Co-founder, Board Chair
Co-founder, Home Biosciences
Board Mb Sequantrix, One Biosciences
CEO, Ariceum
Former IPSEN, DBV

Vanessa Malier
Board Member
Chairwoman of the Board, Nuage Tx
Advisor to biotechs, VC and PE funds
Former Kurma Partners and IPSEN

Pascale Auge PhD
Board Member
Chairman of the Exec Mgt Board, Inserm Transfert
Board Member, LFB.
Former Neurotech, Entomed, ABScience

Dr. Magali Richard
Co-founder, Board Member
Co-founder, Home Biosciences
Board Mb, One Biosciences
HP Community Lead, Alan
Former DBV, BCG
Founders & board members
ENDOMETRIOSIS
190 million women affected worldwide. A massive gap in need of an adequate response
~190M
Women affected globally
10%
Of reproductive-age women (1)
50%
Of infertile women (2)
The Disease

Debilitating
inflammatory disease
-
Ectopic endometrium
-
Dysmenorrhoea, chronic pelvic pain, dyspareunia, proctalgia, dysuria
-
Fatigue, depression
-
3.61 higher odds of infertility (3)

Economic disaster
-
$78–119bn in the US (5, 6)
-
Cost to UK economy £8.2bn in Tx, loss of work and HC costs (4)
%201_edited_edited.jpg)
Current treatments inadequate
-
NSAIDs (limited efficacy)
-
Hormonal treatments (side effects)
-
Surgery (high recurrence rates)
-
No disease-modifying therapies available
Role of PIK3CA in Endometriosis



Up-regulation of PIK3CA in endometriosis

PKI3CA is a major target in endometriosis
Mutation driven : ca 1/4 patients
Non-mutation driven : ca 3/4 patients
-
Loss / inactivation of PTEN
-
KRAS mutation
-
TKR activation
ST420
Uniquely designed for Endometriosis
COMPANY
COMPOUND / BRAND
DVT PHASE
INDICATIONS
MUTANT
Apikal Therapeutics
ST420
PC
Endometriosis
O
O
Eli Lilly
Eli Lilly (abandonné)
Ensem Therapeutics
Laekna Therapeutics
OnKure Therapeutics
Relay Therapeutics
Scorpion / Lilly
Totus Medicines
ARTham Tx / Kaken
Faeth Tx
Novartis
Novartis
Novartis
Roche / Genentech
NO
LY4045004
PC
LOXO-783
1
Cancers H1047R
ETX-636
1
PIK3CA mutant tumors
LAE118
1
PIK3CA mutant tumors
OKI-219
1
Cancers H1047R
RLY-2608
1
PIK3CA mutant tumors
STX-478
1
PIK3CA mutant tumors
TOS-358
1
PIK3CA mutant tumors
Serabelisib (ART-001, TAK-117)
2
PIK3CA-related vasc malformations (SFVM : MV, ML, KTS)
Serabelisib (ART-001, TAK-117)
2
Advanced / Relapsing endometrial cancer
Alpelisib (BYL719) / Piqray
2
Ovarian Cancer w PIK3CA mutations
Alpelisib (BYL719) / Piqray
Approved
Breast K HR+/HER2-, Metastatic, PIK3CA mutant
Alpelisib (BYL719) / Piqray
Approved
PROS
Inavolisib (GDC-0077) / Itovebi
Approved
Breast K HR+/HER2-, PIK3CA mutant
O
O
O
O
O
O
O
O
O
O
O
O
O
O
N
N
N
N
N
N
N
TBD
O
O
O
O
O
O
NO
LOW
LOW
LOW
NO
LOW
LOW
LOW
LOW
LOW
Y
Y
Y
Y
WT
HYPER-GLYCEMIA
PIK3CA INHIBITION

ST420’s unique PIK3CA safety profile enables use in endometriosis
Controlled Glucose and Insulin Levels
Glucose levels (mmol/l)
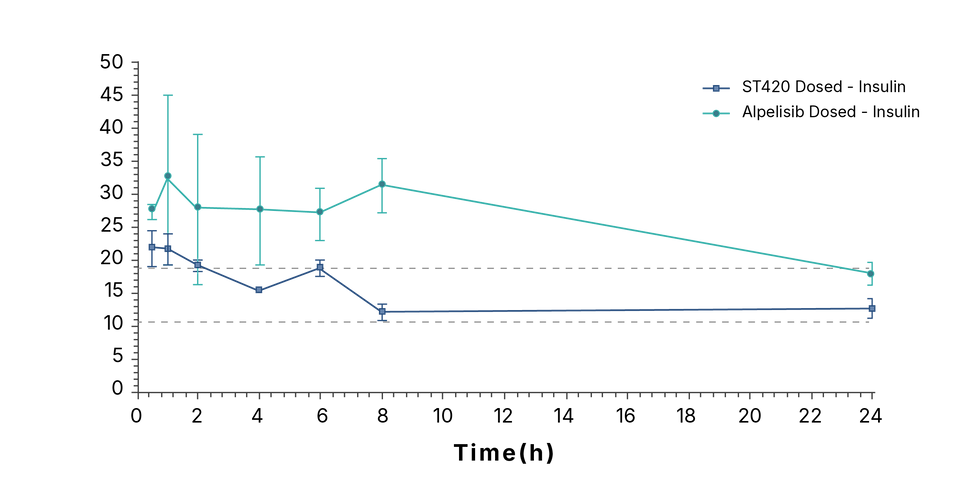
Insulin levels (ng/ml)

-
Selvita CRO, Jan 2024
-
Mice treated for 6 days
-
Glucose and insulin levels measured
Results reproduced independently in multiple models (mice and rabbits) by Pr. Canaud’s team and CROs (Eurofins, Selvita) – with consistent and confirmatory results
Development pathway
Significant development derisking
-
PIK3CA activity is established
-
Glycemia & Tox profile clarified
-
No major CMC hurdle
30 M€ Series A
-
10 M€ to First in Man
-
20 M€ to Phase 1 POC
-
Execute pre-clinical and Ph1 plan to strongest inflexion point : Confirmed activity and glycemic profile in patients.
![20260113_apikal infographic[C]-04.png](https://static.wixstatic.com/media/ea90ad_0bf16efc52a54ab18b811e10746835f6~mv2.png/v1/fill/w_980,h_371,al_c,q_90,usm_0.66_1.00_0.01,enc_avif,quality_auto/20260113_apikal%20infographic%5BC%5D-04.png)
Pipeline
![20260113_apikal infographic_[3c]-03.png](https://static.wixstatic.com/media/ea90ad_ac50896c28b7420c8461107b14850f54~mv2.png/v1/fill/w_980,h_680,al_c,q_90,usm_0.66_1.00_0.01,enc_avif,quality_auto/20260113_apikal%20infographic_%5B3c%5D-03.png)
CONTACT US
Get in touch
Interested in learning more about Apikal Therapeutics and ST420?
We’d love to hear from you.
Phone 1234567890


Developing breakthrough therapies for endometriosis and other chronic immuno-inflammatory disease.
© 2025 Apikal Therapeutics. All Rights Reserved.
